
Read more about periodic trends here: brainly.
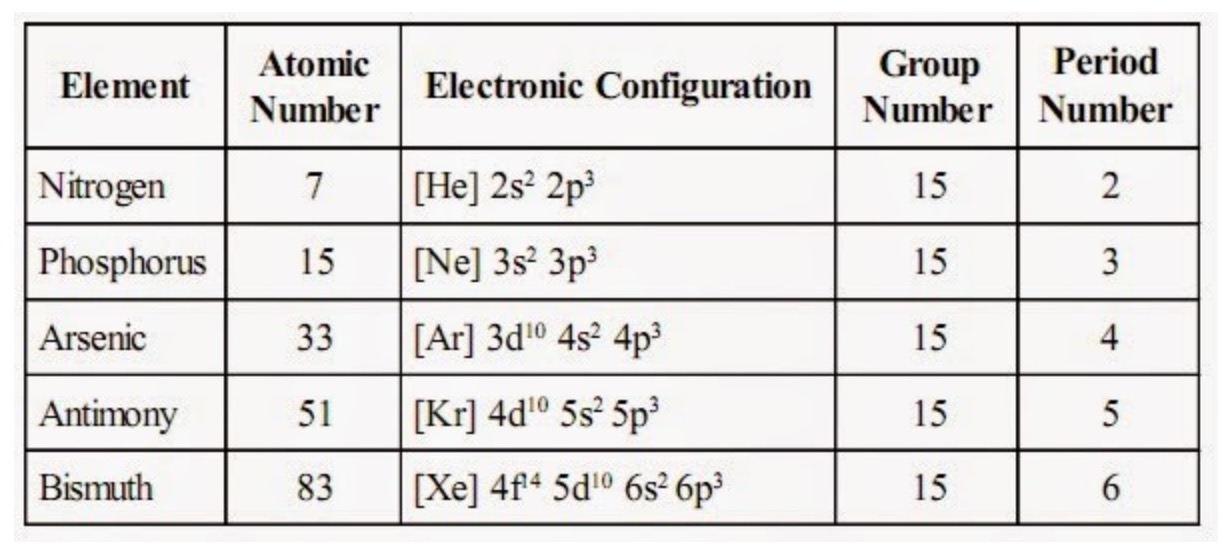
Since Antimony belongs to group 15 and period 5, its valence electrons is 5. This is an application of Equations 7.2.1 and 7.2.2. Ions is the term for atoms that have gained or lost electrons.Īs said earlier, looking at the periodic table can help us know the number of valence electrons of an atom. Each species has 10 electrons, and the number of nonvalence electrons is 2 (10 total electrons - 8 valence), but the effective nuclear charge varies because each has a different atomic number A. That is why it is "electrically neutral" because the atom hasn't gained or lost any electrons. In these groups and periods, elements are together because of their similarities like number of valence electrons, electron affinity, and electronegativity.Įlectrically neutral atoms are atoms that contains no net charge. Elements in the periodic table are arranged in a manner where you can find them in groups and periods. One way to know the number of valence electrons present in an atom is to look at the periodic table and periodic trends. The first is to use the Periodic Table to figure out how many electrons Antimony has in its valence shell. Bond order refers to the number of bonds that can be formed between two atoms. There are two ways to find the number of valence electrons in Antimony (Sb). Valence electrons also indicate the bond order of a chemical compound.
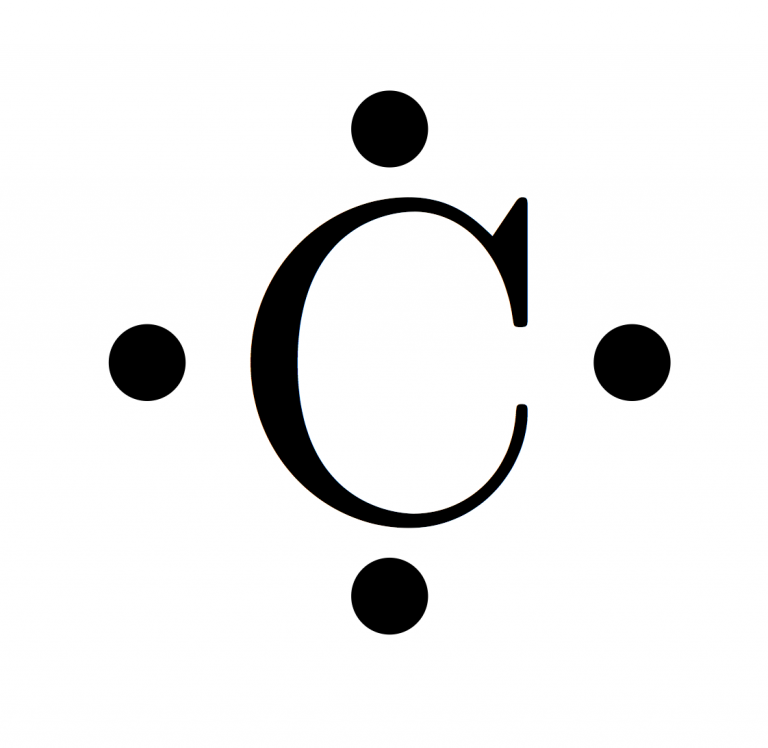
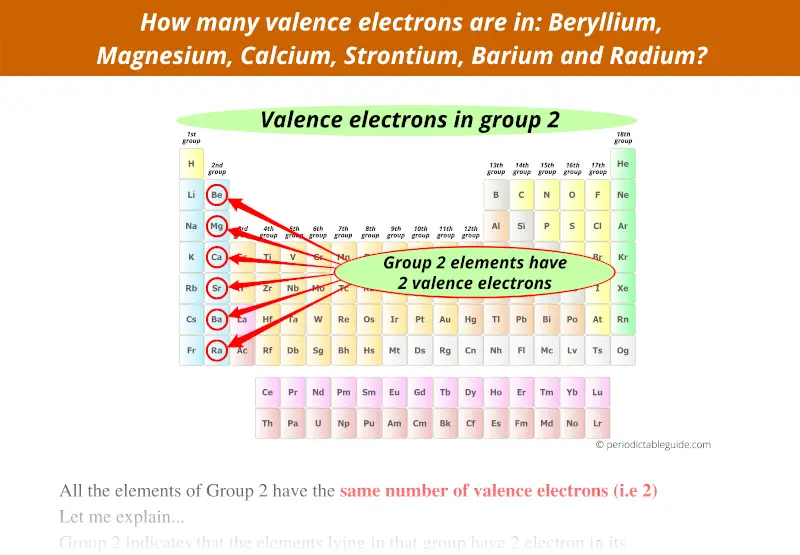
They are considered important because they are responsible for some of the element’s chemical properties like the element's electronegativity. Valence electrons are electrons that lie at the outermost shell of the nucleus' electron orbit. The number of valence electrons in of an electrically neutral antimony atom is 5.


 0 kommentar(er)
0 kommentar(er)
